Understanding the Definitions of Ionization Energy and Electron Affinity
Release Does the following reaction absorb or. In other words the neutral atoms likelihood of gaining an electron.
Solved Understanding The Definitions Of Ionization Energy Chegg Com
Below are the key differences between electron affinity and electronegativity.

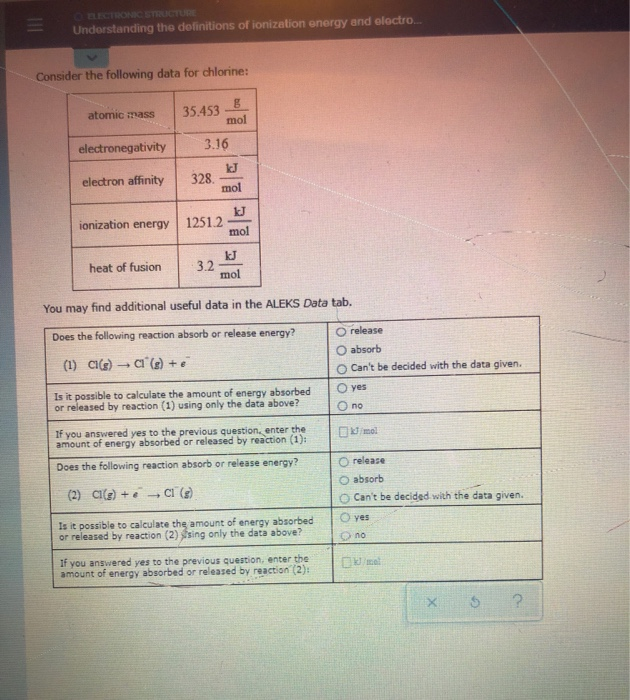
. From the internet. Understanding the definitions of ionization energy and electron. Mol ionization energy 12512 mol heat of fusion 32 You may find additional useful data in.
Cation Atoms at the TOP BOTTOM. Atomic mass 74922 mol electronegativity 218 electron affinity kJ 78. Similarly electron affinity decreases from top to bottom down the table just as ionization energy does.
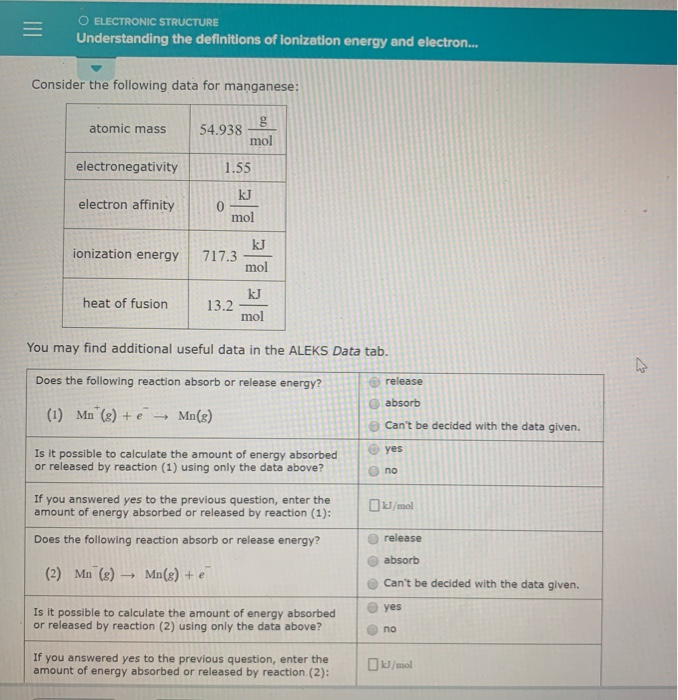
O ELECTRONIC STRUCTURE Understanding the definitions of ionization energy and electron. Electron affinity is defined as the change in energy in kJmole of a neutral atom in the gaseous phase when an electron is added to the atom to form a negative ion. Atomic mass g 39098 mol electronegativity 082 electron affinity kJ 484 mol ionization energy KJ 4188 mol kJ heat of fusion 233 mol You may find additional useful data in the ALEKS Data tab.
The main difference between electron affinity and ionization energy is that electron affinity gives the amount of energy released when an atom gains an electron whereas ionization energy is the amount of energy required to remove an. Ionization reactions always absorb energy called the ionization energy IE. Ionization energy is effectively a measure of how easy or hard it is to convert a neutral atom into a CATION ANION.
The change in energy in kJmole of a neutral atom or molecule in the gaseous phase when an electron is added to the atom to form a negative ion. The electron affinity of an atom increases along the periods on the table just as ionization energy also increases. Understanding the definitions of energy and electron affinity Problem.
Ionization energy is related with making cations from neutral atoms and electron affinity is related with. In other words it can be expressed as the neutral atoms likelihood of gaining an electron. Does the following reaction absorb or release energy.
This property is a fixed and measurable value. It is the minimum amount of energy required to remove the most loosely bound electron of an isolated gaseous atom or ion. First electron affinity the energy required to add one mole of electrons to one mole of gaseous atoms second electron affinity the energy required to add one mole of electrons to one mole of gaseous 1- ions Electronegativity - This is a different concept altogether.
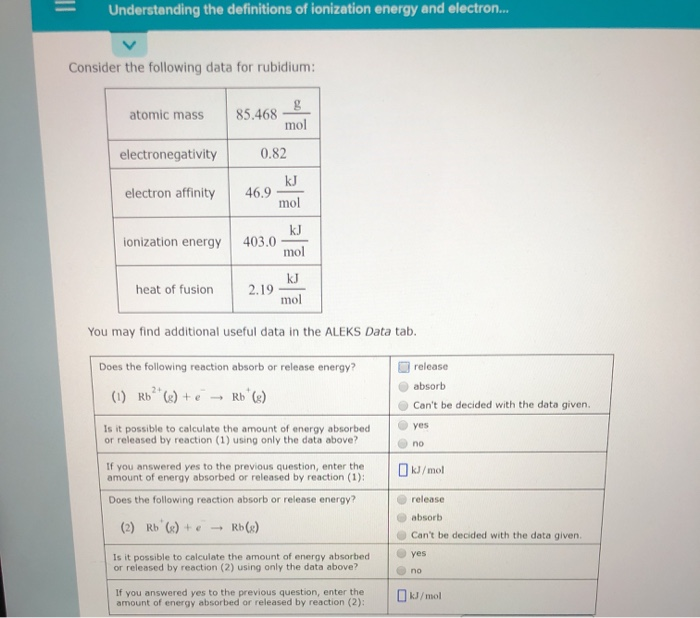
Electron affinities are more difficult to measure than ionization energies. Consider the following data for potassium. O ELECTRONIC STRUCTURE Understanding the definitions of ionization energy and electron.
Electron affinity and ionization energy are two chemical terms used to describe the behavior of electrons and atom quantitatively. In chemistry and atomic physics the electron affinity of an atom or molecule is defined as. X e X energy Affinity H.
Atomic mass 11871 g mol electronegativity 196 electron affinity 1073 kJ mol ionization energy kJ 7086 mol heat of fusion KJ 70 mol You may find additional useful data in the ALEKS Data tab. Can anyone explain these to me in normal English please. Understanding the definitions of ionization energy and electro.
I am having a hard time understanding these concepts from the definitions given in the book and from my teacher. The energy released when an electron is added to a gaseous atom which is in its ground state to form a gaseous negative ion is defined as the first electron affinity. The cause of the decrease in both electron affinity and ionization energy is the same as well the shielding effect.
Atomic mass 354538 electronegativity 316 electron affinity 328. Electron affinity is defined as the quantitative measurement of the energy change that results from adding a new electron to a neutral atom or molecule in the gaseous state. Consider the following data for arsenic.
What is the difference between Ionization Energy and Electron Affinity. Consider the following data for chlorine. Ionization Energy and Electron Affinity.
Its electronegativity is 1 28 2 30 5 3 3. Note that ionization energies measure the tendency of a neutral atom to resist the loss of electrons. 4 40.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Atomic mass 10787 mol 193 electronegativity electron affinity 1256 mol ionization energy 7310 mol heat of fusion 113 mol You may find additional useful data in the ALEKS Data tab. Electron affinity Electronegativity Ionization energy 1.
A reaction in which a neutral atom loses an outer electron e- on the right side of the equation is called an ionization reaction. Electro Negativity Ionization Energy and Electron Affinity. Electron affinity of an atom or specie is defined as the amount of energy released or absorbed when an electron is attached to the atom or ion.
Mol ionization energy 9470 kJ mol heat of fusion KJ 277 mol You may find additional useful data in. Electron affinity is defined as the amount of energy liberated when a molecule or neutral atom acquires an electron from outside. Consider the following data for silver.
Understanding the definitions of ionization energy and electron. Consider the following data for tin. Ionization energy is the amount of energy needed to remove an electron from a neutral atom.
The more negative the electron affinity value the higher an atoms affinity for electronsThe energy of an atom is stated when an atom loses or gains energy through. Objectives Examine periodic trends in ionization energy Examine periodic trends in electron affinity Key Terms Ionization energy Electron Affinity Ionization Energy Ease at which electrons can be removed from an atom or ion First ionization energy I1 is the energy required to remove the first electron from neutral atom. Click hereto get an answer to your question 56 174 The ionisation energy and electron affinity of an element are 130ev and 38ev respectively.
Electron affinity is the amount of energy released when electron is added to an atom. Hence the definition also depends on what you are adding electrons to.

8 3c Understanding The Definitions Of Ionization Energy And Electron Affinity Youtube
Solved Understanding The Definitions Of Ionization Energy Chegg Com
Solved O Electronic Structure Understanding The Definitions Chegg Com
No comments for "Understanding the Definitions of Ionization Energy and Electron Affinity"
Post a Comment